

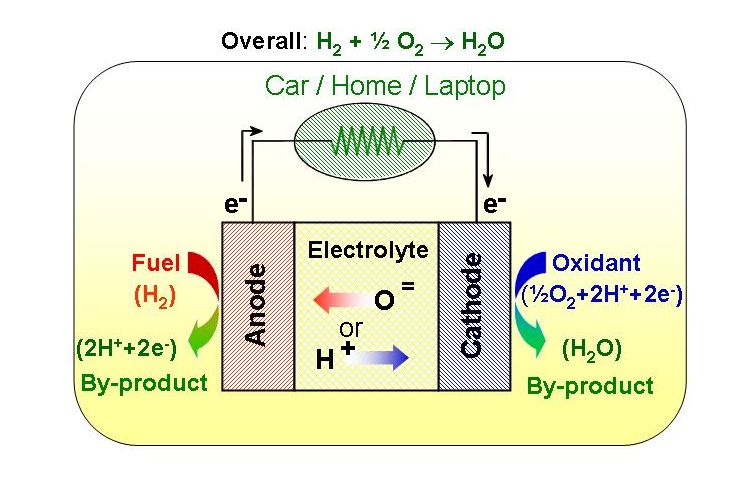
 |
In a simple fuel cell, hydrogen fuel is delivered to the anode of the fuel cell. Oxygen (usually from air) is delivered through the cathode on the other side of the membrane. Passing through a catalyst, hydrogen atoms split into protons and electrons (H2 → 2H+ + 2e-). The protons are transported through the electrolyte, while the electrons are harnessed and diverted out of the fuel cell to provide electric power to a device. The electrons ultimately reunite with the protons at the cathode in the presence of oxygen gas and a catalyst to generate water, which is then expelled (½O2 + 2H+ + 2e- → H2O). A fuel cell system which includes a "fuel reformer" can utilize the hydrogen from any hydrocarbon fuel, i.e., from natural gas to methanol to even gasoline.
Fuel cells thus combine the advantage of battery technology with the advantage of combustion engines: like batteries, they operate by having very well controlled electrochemical reactions (which accounts for their high fuel efficiency); and like combustion engines, they can be refuelled.
Table 1. Common types of fuel cells, their temperature of operation, and electrolyte used.
| Fuel Cell Type | Temperature | Electrolyte |
| SAFC - Solid Acid | 200-300°C | Solid acids, e.g. CsHSO4 |
| PEMFC - Polymer Electrolyte Membrane | 70-100°C | Sulfonated polymers, e.g. Nafion® |
| AFC - Alkaline | 100-200°C | Aqueous KOH |
| PAFC - Phosporic acid | 150-200°C | H3PO4 |
| MCFC - Molten carbonate | 600-700°C | (NA,K)2CO3 |
| SOFC - Solid oxide | 700-1000°C | (Zr,Y)O2-3 |
A set of Polarized Light Microscope images of the Superprotonic Phase Transition
|
1. Room temperature 2. Beginning transition on left 3. Superprotonic on left side 4. More superprotonic on left side 5. Fully superprotonic 6. Reverse transitioned |
In the case of CsHSO4, the bisulfate (HSO4-) group forms a tetrahedron with an oxygen atom at each corner and a hydrogen atom sitting on one of the oxygens. At room temperature, all the sulfate groups have a fixed orientation. When the temperature is raised, disorder sets in and the sulfate groups reorient, changing the positions of the hydrogen atoms as they do so. The time frame for this reorientation is about 10-12 seconds. Occasionally, a proton from one sulfate group transfers over to the next, with a transfer rate on the order of 109 Hz. Essentially, these sulfate groups rotate almost freely - and every 1000 reorientations or so, they're in exactly the right position for a proton transfer to happen. As the material goes through this transition, there's a sudden increase in conductivity of several orders of magnitude. Conductivity values for the acid salts are comparable to the conductivity of Nafion and other polymer electrolytes, but at moderately higher temperatures. A number of different solid acid compounds with such behavior have been discovered.
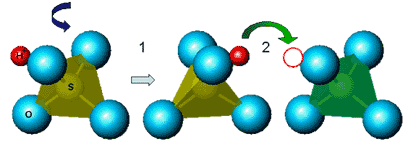 |
Figure 2. Proton conduction mechanism for solid acids (CsHSO4 shown here). Protons (H+) attached to sulfate tetrahedra are rapidly repositioned (1011Hz) by rotations of the tetrahedra (1). Approximately once every one hundred rotations (109Hz), the proton finds itself in an ideal configuration to hop onto a neighboring tetrahedra (2).
 |
 |
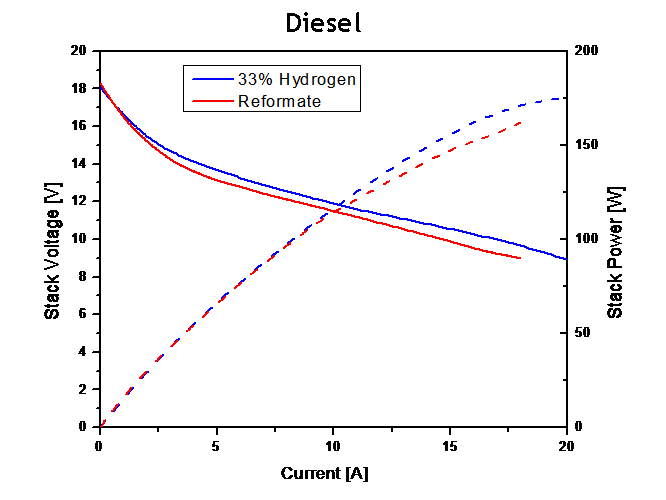 |
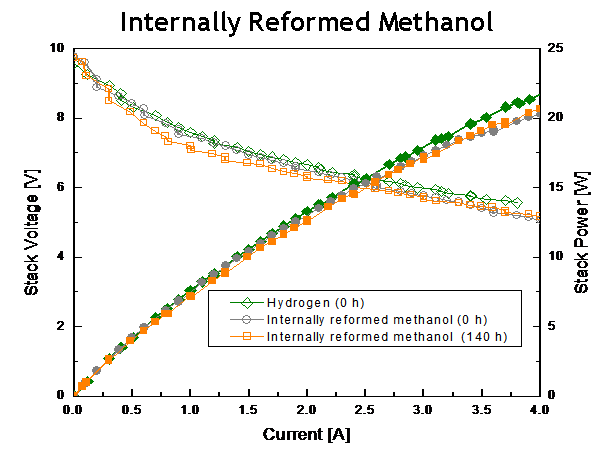
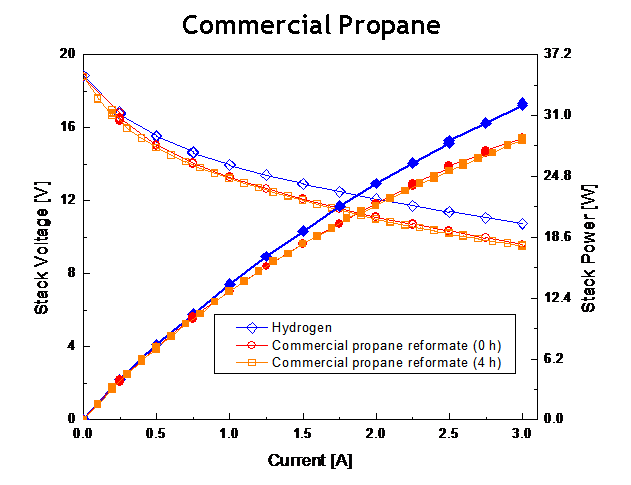
Figure 3. SAFC stack performance utilizing a) internally reformed methanol, b) commercial propane (Home Depot brand) and c) diesel (STATOIL fuel station, tested at Nordic Power Systems) all versus Hydrogen baseline
Home • About Us • Our Technology • In the Press • Contact Us
© Copyright 2014 Safcell, All Rights Reserved.